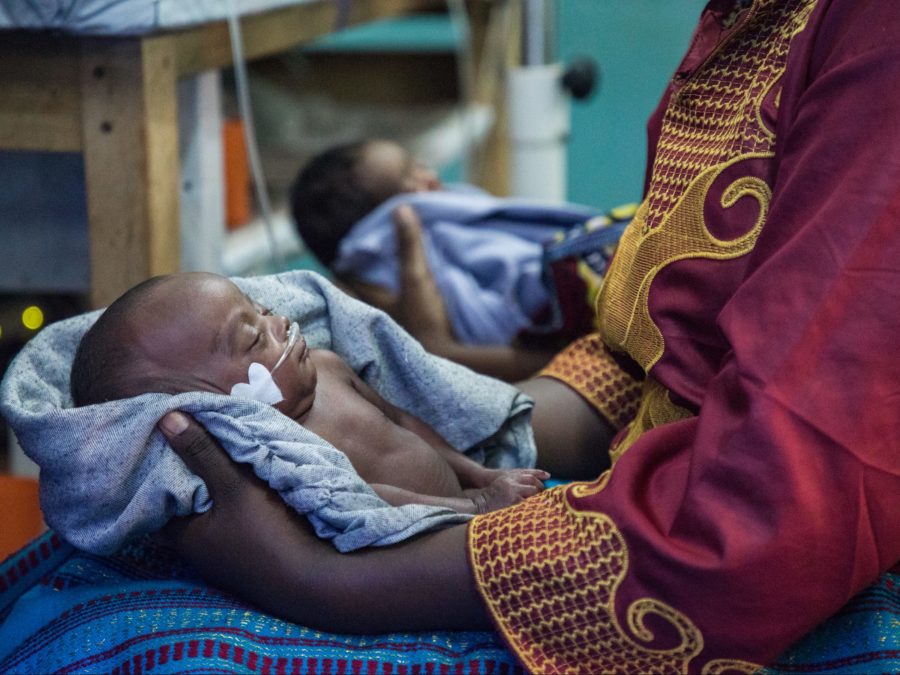
Neonatal sepsis
Children and newborns are especially vulnerable to drug-resistant bacteria. 1 in 5 deaths caused by antibiotic resistance occur in children under the age of five, and up to 3 million newborns get serious infections that lead to sepsis every year. GARDP is working to identify and develop new treatments for children, with special attention to newborns with sepsis, as well as to provide data to inform treatment guidelines for this vulnerable group. This page focuses on sepsis in newborns; for information on our paediatric development work, please see this page.

Programme goals
Identify and improve access to new treatment regimens for newborns with life-threatening sepsis

Contribute data to inform global guidelines for effective antibiotic use in newborns and children
In line with 2024-2028 GARDP Strategy, develop new treatments for carbapenem-resistant infections in newborns
“If we don’t carry out clinical trials specifically for children—as difficult as they may be to carry out—doctors won’t have the evidence they need to treat children, especially newborns. You can’t make assumptions based on adult data to treat a baby. With more data, we can identify the right drug at the right dosage to treat newborns with sepsis.”
– Sally Ellis, Children’s Antibiotics Project Leader, GARDP
Current projects
Trial of new and existing treatments for sepsis in newborns
GARDP is running a trial (“NeoSep1”) to validate the doses of two antibiotics (fosfomycin and flomoxef) for use in newborns. It will also rank the safety and efficacy of three new combinations of older antibiotics (fosfomycin-amikacin, flomoxef-amikacin, and flomoxef-fosfomycin) against the current WHO-recommended standard of care (ampicillin-gentamicin). The trial will also consider how these combination treatments can best be used in hospital settings with varying levels of antibiotic resistance. The initial phase of the trial is taking place at hospitals in Kenya and South Africa. It will be expanded to other countries and regions with the aim to enrol 3,000+ newborns globally.
The trial (“NeoSep1”) builds on findings from a global observational study of sepsis in newborns carried out by GARDP together with St George’s, University of London; Penta–Child Health Research; the Medical Research Council Clinical Trials Unit at University College London, whose research team led in analysing the data; and the University of Antwerp. As one of the largest ever observational studies on the care of babies with sepsis, the study looked at over 3,200 newborns at 19 sites in 11 countries on 4 continents. It found that newborns with neonatal sepsis are increasingly dying because of drug-resistant bacterial infections. GARDP published the findings in PLOS Medicine in June 2023 and produced a report in 2022.
GARDP also carried out additional research leading up to the trial (“NeoSep1”). Previously, GARDP and its partners conducted a clinical trial that tested the safety and pharmacokinetics (i.e. how a drug interacts with the body) of fosfomycin in babies with neonatal sepsis. The results were published in Archives of Disease in Childhood and the Journal of Antimicrobial Chemotherapy. In addition, GARDP carried out studies in a laboratory that suggested that two older antibiotics in combination could be useful to treat neonatal sepsis and warranted further clinical investigation. See results in the Journal of Antimicrobial Chemotherapy.




Development of new treatments for carbapenem-resistant infections in newborns
Carbapenem-resistant Acinetobacter baumannii (CRAB) and carbapenem-resistant Enterobacterales (CREs) cause infections for which there are very limited or no safe and effective treatment options for children and newborns. With this project, GARDP will strive to improve outcomes for newborns by accelerating the development of new treatments for these drug-resistant infections. It will identify potential regimens for the use of cefiderocol and/or cefepime-taniborbactam for neonatal sepsis, with plans to initiate clinical development by 2028.




Key milestones
- 2019: COMPLETED clinical trial to confirm the safety and pharmacokinetics (i.e. how a drug interacts with the body) of fosfomycin in babies with neonatal sepsis
- 2020: COMPLETED observational study on the care of babies with sepis involving over 3,200 newborns at 19 sites in 11 countries on 4 continents
- 2022: IDENTIFIED three potential combination treatments for neonatal sepsis
- 2023: LAUNCHED trial of new antibiotic combinations to treat neonatal sepsis in Kenya and South Africa




About children’s antibiotics
About children’s antibiotics
The development of new antibiotics is not keeping pace with rising antibiotic resistance. When it comes to developing treatments for children, the situation is even more dire.
According to a recent study, in sub-Saharan Africa in 2019, more than half of the deaths caused by antibiotic-resistant infections occurred in children under five. Worldwide that same year, nearly 140,000 newborns died because of antibiotic-resistant infections, including pneumonia, sepsis, and meningitis. About 40% of infections that cause neonatal sepsis in hospitals are reported to be resistant to standard treatments.
It is not enough to develop new antibiotics based on trials in adults. Children’s bodies respond differently to antibiotics than adults’ bodies, so separate steps must be taken to confirm safety and effectiveness (including the proper dosage) of each new antibiotic treatment in children. Although regulatory agencies require that pharmaceutical companies evaluate new treatments for use in children, such steps are often delayed for years, if undertaken at all.
Doctors and policymakers need more data, treatments, and guidelines to care optimally for newborns and children with bacterial infections. GARDP is addressing this vital need.